Description
What is Keytruda and how is it used?
Keytruda is a prescription medicine used to treat the symptoms of Melanoma and for many other cancer types such as Non Small Lung Cancer, Head and Neck Squamous Cell Cancer, Classical Hodgkin Lymphoma, and Primary Medistinal Large B-Cell Lymphoma. Keytruda may be used alone or with other medications.
Keytruda belongs to a class of drugs called Antineoplastics, Monoclonal Antibody; PD-1/PD-L1 Inhibitors.
What are the possible side effects of Keytruda?
Keytruda may cause serious side effects including:
- shortness of breath,
- chest pain,
- new or worse cough,
- diarrhea,
- more frequent bowel movements than usual,
- stools that are black or tarry,
- severe stomach pain or tenderness,
- yellowing of the skin or eyes (jaundice),
- nausea,
- vomiting,
- stomach pain (upper right side),
- dark urine,
- loss of appetite,
- bleeding or bruising easily,
- rapid heartbeats,
- weight loss,
- weight gain,
- sweating,
- increased thirst or hunger,
- increased urination,
- hair loss,
- feeling cold,
- constipation,
- your voice gets deeper,
- muscle aches,
- dizziness,
- fainting,
- headaches that will not go away or unusual headaches,
- change in the amount or color of your urine,
- rash,
- changes in your eyesight,
- severe or persistent muscle or joint pain,
- severe muscle weakness,
- low red blood cells (anemia),
- chills,
- shaking,
- wheezing,
- itching,
- rash,
- flushing,
- fever, and
- lightheadedness
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Keytruda include:
-
- fatigue,
- cough,
- shortness of breath,
- nausea,
- itching,
- rash,
- loss of skin pigmentation (vitiligo),
- decreased appetite,
- headache,
- constipation,
- joint pain,
- back pain, and
- diarrhea
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Keytruda. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
Pembrolizumab is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. Pembrolizumab is an IgG4 kappa immunoglobulin with an approximate molecular weight of 149 kDa. Pembrolizumab is produced in recombinant Chinese hamster ovary (CHO) cells.
KEYTRUDA for injection is a sterile, preservative-free, white to off-white lyophilized powder in single-dose vials. Each vial is reconstituted and diluted for intravenous infusion. Each 2 mL of reconstituted solution contains 50 mg of pembrolizumab and is formulated in L-histidine (3.1 mg), polysorbate 80 (0.4 mg), and sucrose (140 mg). May contain hydrochloric acid/sodium hydroxide to adjust pH to 5.5.
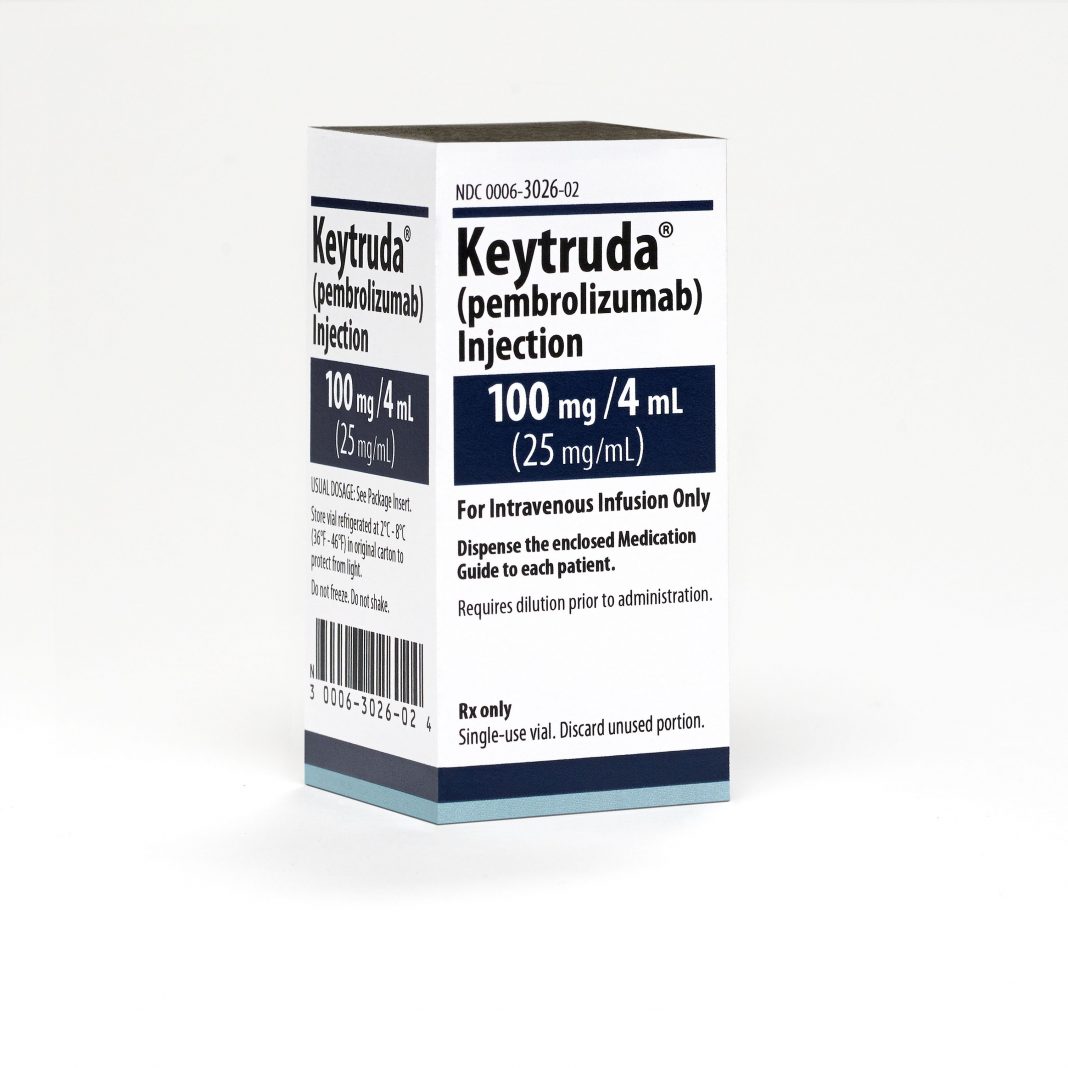
KEYTRUDA injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution that requires dilution for intravenous infusion. Each vial contains 100 mg of pembrolizumab in 4 mL of solution. Each 1 mL of solution contains 25 mg of pembrolizumab and is formulated in: L-histidine (1.55 mg), polysorbate 80 (0.2 mg), sucrose (70 mg), and Water for Injection, USP.




Reviews
There are no reviews yet.